Lab# 21: Balancing Chemical Equations
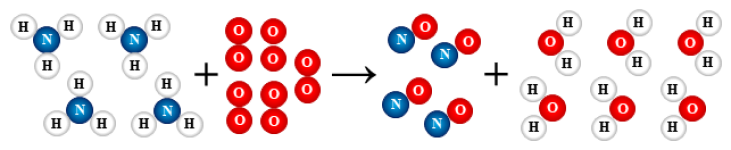
1.
2 H2 + O2 → 2 H2O
synthesis
synthesis

11.
4 Li + O2 → 2 LiO2
synthesis
synthesis

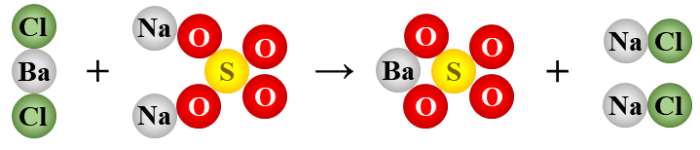
2.
BaCl2 + Na2SO4 → BaSO4 + 2 NaCl
double replacement
double replacement
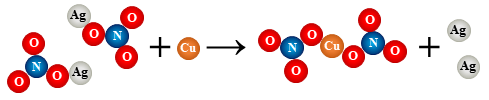
12.
2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
single replacement
single replacement
3.
Si + 2 Cl2 → SiCl4
synthesis
synthesis
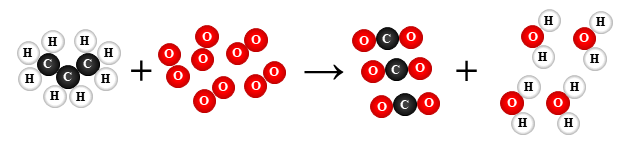
13.
C3H8 + 5 O2 → 3 CO2 + 4 H2O
combustion
combustion
4.
Cl2 + 2 KBr → 2 KCl + Br2
single replacement
single replacement

14.
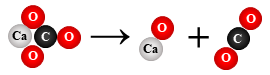
CaCO3 → CaO + CO2
decomposition
decomposition
5.
2 Na + Cl2 → 2 NaCl
synthesis
synthesis
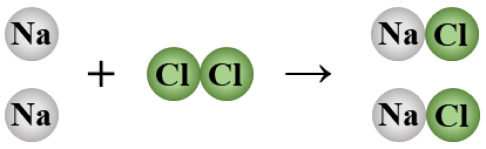
15.
NaOH + HCl → NaCl + H2O
double replacement (neutralization)
double replacement (neutralization)
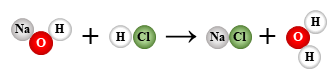
6.
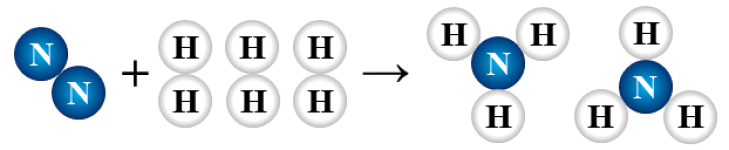
N2 + 3 H2 → 2 NH3
synthesis
synthesis
16.
2 HgO → 2 Hg + O2
decomposition
decomposition

7.
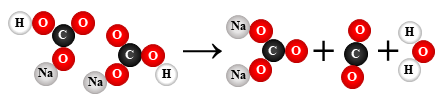
2 NaHCO3 → Na2CO3 + CO2 + H2O
decomposition
decomposition
17.
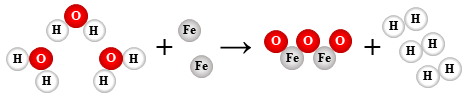
3 H2O + 2 Fe → Fe2O3 + 3 H2
single replacement
single replacement
8.
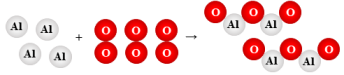
4 Al + 3 O2 → 2 Al2O3
synthesis
synthesis
18.
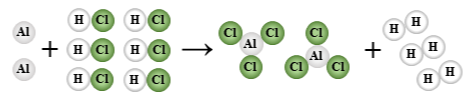
2 Al + 6 HCl → 2 AlCl3 + 3 H2
single replacement
single replacement
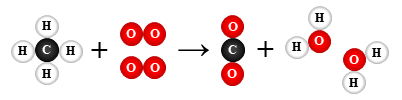
9.
CH4 + 2 O2 → CO2 + 2 H2O
combustion
combustion
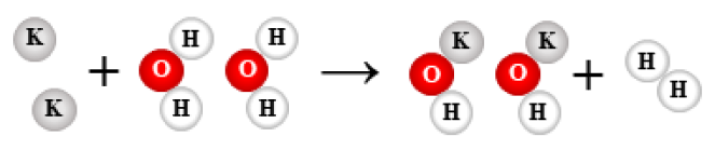
19.
2 K + 2 H2O → 2 KOH + H2
single replacement
single replacement
10.
2 HCl + Zn → NaCl2 + H2
single replacement
single replacement

20.
4 NH3 + 5 O2 → 4 NO + 6 H2O
combustion
combustion